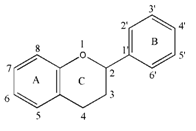
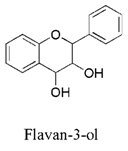
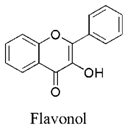
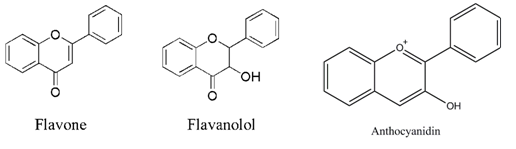
Introduction:
Sunlight comprises of electromagnetic radiation of various wavelengths which include wavelengths from UV to visible range. In the recent years, there has been a rise in the magnitude of ultraviolet light reaching the earth surface due to the fact that stratospheric ozone is being depleted at a fast rate, which in turn has led to increased cases of skin cancer and other skin disorders. Solar UV radiation is grouped in 3 classes based on their wavelengths:
- UV-A (320-400 nm)
- UV-B (290-320 nm)
- UV-C (200-290 nm)
UV-C radiation is filtered by the ozone layer whereas UV-A radiation penetrates deeper into the layers of the skin and is barely able to stimulate the DNA molecule directly and exerts its mutagenic and carcinogenic action through oxidative stress. If UVB is directly absorbed it leads to direct disruption of DNA, which produces photoproducts like cyclobutane pyrimidine dimers (CPD) and pyrimidine-pyrimidone dimers, which if remained un-repaired may commence photo-carcinogenesis. Thus protection against the radiations is vital for thwarting the damage. Dermatological preparations like sunscreens are available for providing a defense barrier to the skin. These preparations contain organic or inorganic filters which act as a medium for filtering the radiations. [1,8]