- Introduction
Carbamazepine contains dibenzepine ring system which is structurally related to the tri-cyclic antidepressants [1–4] specifically used in therapy for seizures. Carbamazepine is highly insoluble in water at ambient temperature (25ºC) [5–8] with a solubility of 170mg/l. As the Carbamazepine is representing low water solubility, its bioavailability is also very low. Also polymorphism is observed for carbamazepine, which may affect its solubility. Upon multiple dosing of carbamazepine, it gets transformed to its metabolite and its half-life (t1/2) is decreased [9]. The research says that central nervous system (CNS) side effects, related with immediate-release formulation of carbamazepine were reduced when patients were moved to an extended-release formulation [10]. So, sustained release formulation of carbamazepine is most effecting in maintaining the concentration in systemic circulation [9].
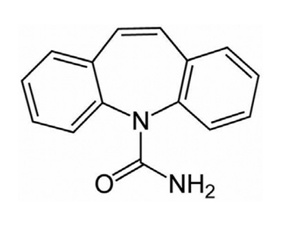
Figure-1 Carbamazepine Chemical Structure
Literature described the transformation of one polymorphic form to another dihydrate form, which is showing variable dissolution