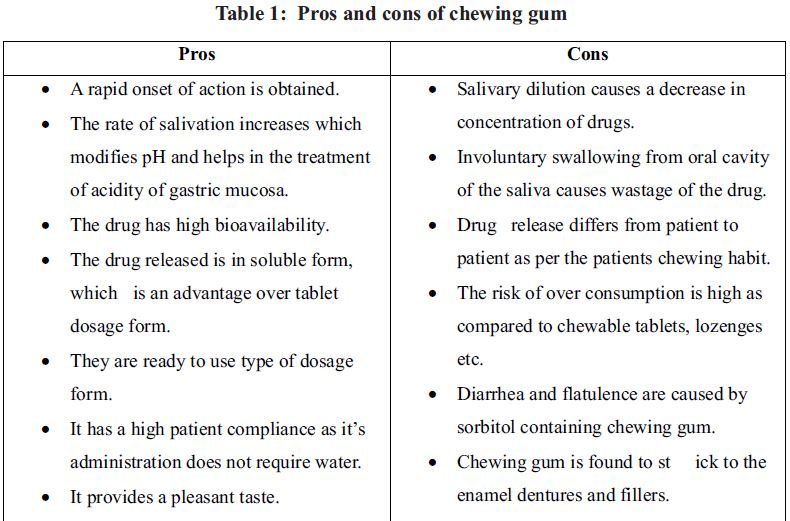
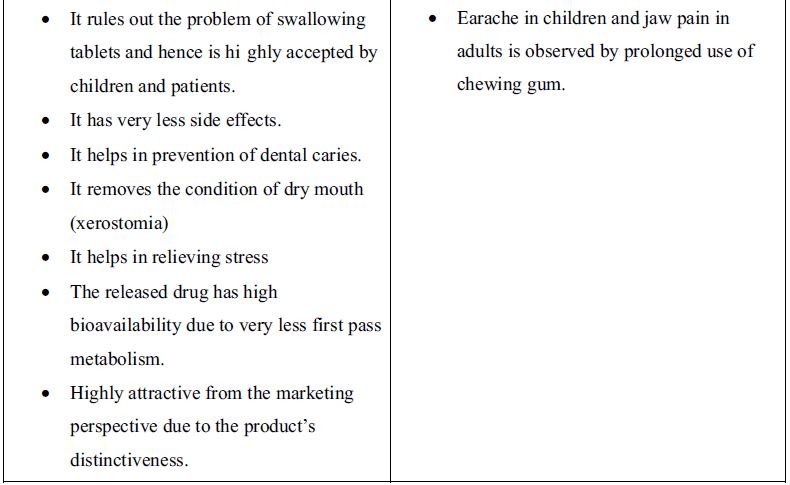
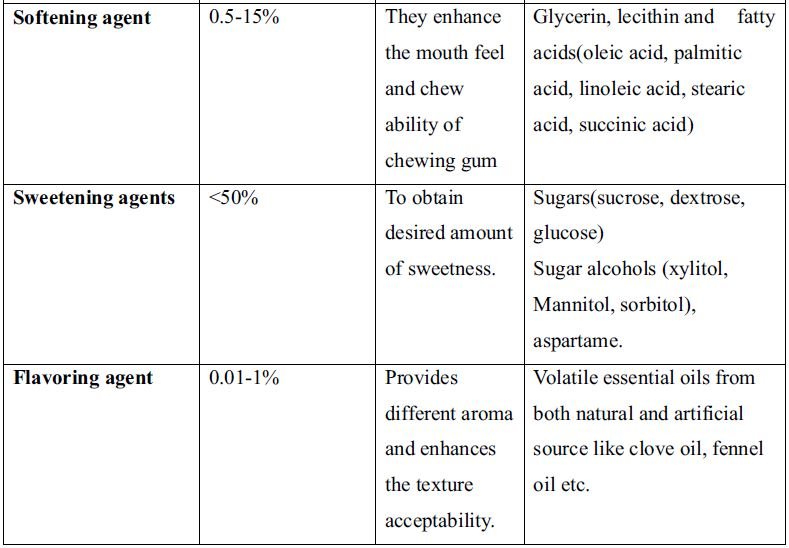
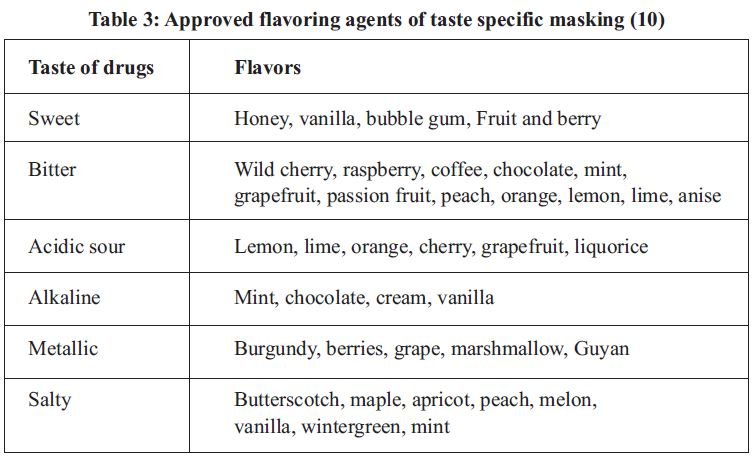
1. Introduction
Chewing gums are the agents which offer an alternative as well as a novel drug delivery system (1). Chewing gum have an old and long history (2). The Greeks used mastic, a resin from the bark of mastic tree for cleaning their teeth and improving the smell of breath. The Mayan Indians obtained gum from the sapodilla tree, a member of the family Sapotaceae for the same purpose (3). Due to certain limitations and the shortage of the natural gum, this paved out the way for synthetic gum during the period of World War 2 (4). Then after at the end of world war i.e. in the year 1948 for the first time chewing gum was marketed and commercialized by the name “state of Maine pure spruce gum”(4). The first medicated chewing gum (MCG) containing an analgesic, acetylsalicylic acid (aspirin) was marketed in the 1928 by the name “aspergum” (5). However, it was not accepted in public domain un till 1978. With the introduction of nicotine chewing gum in the 1980s, the chewing gum begins to be accepted for use (6). Another
chewing gum dimenhydrinate was commercially available for the treatment of motion sickness. For the prevention and treatment of various
oral diseases, an alternate class of antimicrobials comprising of naturally
occurring antimicrobial peptides have been developed in the form of MCG. The aim of formulating such MCG was to effectively delivery and maintain a sufficient antibacterial dose within the oral cavity.